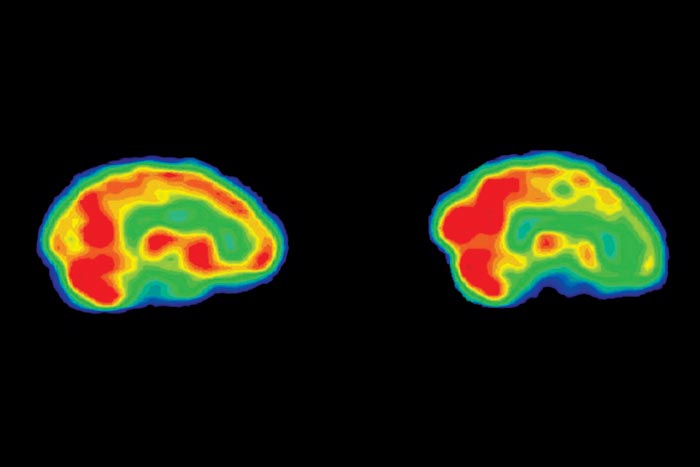
On October 29, 2003, a tall, dark-haired man suffering from severe depression lay on a narrow bed in the PET scan room at the Clarke Institute of Psychiatry in Toronto while a nuclear-medicine technologist injected a small amount of radioactive sugar into a vein in his left arm. He rested quietly in the darkened room as the sugar tracer circulated through his bloodstream and accumulated in the most active regions of his brain. A white sheet of stiff plastic, softened in a steam tray, was then placed over his face and allowed to harden into a mask that would help to keep his head still during the positron emission tomography scan (PET for short) that was about to take place.
The technologist punched the remote and the bed slid back until the man’s head was properly positioned in the doughnut hole of the PET machine. His head was now encircled by a thick ring containing thousands of small crystal detectors that would measure the radiation emitted by the sugar molecules in his brain. He felt anxious and a little claustrophobic, but eventually he relaxed and signalled that the photo session could begin. During the next thirty minutes the PET camera took snapshots showing the levels of activity in different parts of his brain, a process that allows the neurologist to observe the brain in action over time. These images would highlight the most active regions in brilliant red or yellow tones, while less active areas would appear green, blue, or black. They would also reveal patterns of abnormal brain activity, a metabolic signature characteristic of major depression.
Patient X is one of about five million Canadians who will suffer from depression during their lifetimes. He is participating in a study conducted by the neurologist and world-renowned researcher Dr. Helen Mayberg. Mayberg, who was head of neuropsychiatry at Baycrest’s Rotman Research Institute and the University of Toronto until a recent move to Emory University in Atlanta, has developed an exciting new approach to an ancient problem that seems almost as perplexing and intractable today as it did to the classical Greeks. For the past fifteen years, she has been developing a map of the brain to chart the terra incognita of depression. On this odyssey into troubled moods and minds, Mayberg has looked deep inside the brain to expose and illuminate the dark, hidden pathways of depression. Through successive stages of this voyage, she has identified key landmarks that offer fresh approaches to the diagnosis and treatment of one of the commonest and most devastating forms of mental illness.
According to the World Health Organization, depression is the leading cause of disability in developed nations, and the second most cited reason for visits to family doctors in Canada. Apart from the incalculable anguish it causes in the private lives of those who suffer from it, depression also cuts productivity in the workplace and costs the Canadian economy an estimated $14 billion a year. In the past four years alone, the number of antidepressant prescriptions dispensed annually in Canada has increased by nearly fifty percent to more than thirty million, and two leading antidepressants, Paxil and Zoloft, are among the ten top-selling drugs worldwide.
Despite advances in drug treatments and the proliferation of talk therapies, only about one-third of people who suffer from depression seek treatment, and fewer than half of those who receive treatment recover. “Depression is inescapable negativity. You’re in a pit. You can see yourself, and there is no other. The outside world doesn’t exist. How you feel and your sense of self are one. There is no border. It’s all black,” says Helen Mayberg, noting that depression involves disturbances of both mind and mood. “Many people are sad. But what brings a lot of people to the doctor is the fact that they can’t think straight.”
In his depression memoir, Darkness Visible, the novelist William Styron talks about the inexplicable nature of depression and the difficulty of treating it. He recalls a clinician in the field telling him: “If you compare our knowledge with Columbus’s discovery of America, America is yet unknown; we are still down on that little island in the Bahamas.”
The quest to make the great dark mystery of depression visible and treat melancholy moods began about 2,500 years ago with the Greek physician Hippocrates, who, in the late fifth century B.C., was the first to describe a condition he called melancholia. In ancient Greece, human disease was explained in terms of an imbalance of the four humours that were thought to determine human personality. A melancholic, for instance, was considered to have an excess of black bile. Hippocrates observed that melancholics suffered from a lack of appetite, dejection, and insomnia, symptoms we would recognize today. And then, as now, treatments fell into two main categories: medical or physical procedures and therapeutic “lifestyle” prescriptions. The main physical treatments recommended were bloodletting and purgative medicines, intended to eliminate the excess of black bile. But patients were also advised to adopt a healthy regimen: nourishing food, rest, warm baths, support from friends, and amusing activities.
And that was pretty much how things remained until relatively recently. In The Anatomy of Melancholy, the classic Renaissance study of the blues published in 1621, Robert Burton was still recommending the bloodletting and purgatives, while also counselling what we would call psychological treatments: advice and comfort from friends or a doctor, soothing music, mirth, and merry company.
In the early twentieth century, melancholia was finally relabelled depression. The two main streams of therapy (the biological/physical and psychological/talk) continued to evolve, but at a more rapid rate, to become what amounts to a massive trial-and-error experiment. By the late twentieth century, this had produced a bewildering array of treatments.
Modern psychotherapy began at the turn of the century with Sigmund Freud’s development of psychoanalysis and Adolf Meyer, psychiatrist-in-chief at Johns Hopkins Hospital, who understood depression as a reactive rather than a biological disorder and trained a generation of American psychiatrists to look at patients’ life histories and experiences as the keys to understanding and modifying their behaviour. Today, patients can choose from about two hundred types of psycho-therapy ranging from cognitive behaviour therapy to interpersonal therapy, which involve a commitment of time and energy.
Biological/physical treatments of depression offer the magical promise of a quick fix, but here, too, evolution has followed an experimental and sometimes cruel path. In the 1930s, electroconvulsive therapy was introduced as a treatment for severe depression. Crude methods and abuses produced bone fractures, memory loss, and severe brain damage in some patients. In 1936 Dr. Egas Moniz, a Portuguese neurosurgeon, invented the frontal lobotomy, a procedure in which he used a special wire knife to sever white fibres connecting the frontal lobes to the rest of the brain. An American neurologist modified this into a quicker, cruder, and more gruesome procedure, known as the “ice-pick” lobotomy. Frontal lobotomies resulted in zombie-like patients. In the 1940s and 1950s more than 50,000 Americans with mental illness were subjected to lobotomies, with no solid evidence of any benefits.
The 1950s saw a revolution in the biological treatment of depression with the introduction of the first effective antidepressant drugs. A drug called Imipramine was tried as a treatment for hay fever, Parkinson’s disease, and then schizophrenia. Imipramine didn’t control schizophrenia, but it brightened the mood of patients and became the first of the tricyclic class of antidepressants that are still used today. Similarly, Iproniazid, developed to treat tuberculosis, became the first of a second class of antidepressants known as monoamine oxidase inhibitors. As our understanding of depression advanced, a third generation of antidepressants, the most famous of which is Prozac, was developed more purposefully. The first SSRI (Selective Serotonin Re-Uptake Inhibitor) was a compound that boosted the brain chemical serotonin. The big advantage of Prozac, launched in 1987, was that it had fewer side effects than other antidepressants. The commercial success of Prozac spawned newer SSRIs like Paxil, Zoloft, and Celexa, and now there are dozens of antidepressants to choose from in a variety of categories.
But we still don’t know why antidepressants work for some people and not for others. Studies show that cognitive behaviour therapy and antidepressants are about equally effective, helping only about fifty percent of depressed patients. But no one knows for sure who should get drugs or talk therapy, or what drug to try first. For a depressed person seeking the best remedy, there is no clear, objective yardstick to guide the choice of treatment. “The big bugaboo of psychiatry is that there is no reliable, measurable, objective way to accurately predict how someone will do on a particular treatment,” says Mayberg. “In psychiatry, and in depression, there are those who think everyone should get drugs and in another camp there are those that think no one should get drugs. We don’t do this in any other field of medicine. With a heart attack we don’t flip a coin to see whether you are treated with bed rest or a bypass. We have evidence-based rules based on tests like an angiogram. My hope is that in the future we will diagnose and make treatment plans for depression based on the organ that’s injured—the brain.”
Dr. Helen Mayberg did not plan to devote her scientific career to the study of depression. Although she majored in psychobiology as a UCLA undergrad and was fascinated by her encounters with psychiatric patients as a medical student at the University of Southern California in the late 1970s, she chose to pursue a career in neurology. “The problem of mental illness fascinated me,” she says, “but the process of diagnosing and treating mental-health problems was fuzzy. I felt that something important was missing. Neurology is very unfuzzy; I liked the order and objectivity of it. There were rules you could apply. In psychiatry, certain rules might seem to apply, but there wasn’t a known map of the brain to guide you. Psychiatry didn’t have an evidence-based playbook.”
In 1982, Mayberg moved from her native southern California to train at the Neurological Institute of New York at Columbia University. There, she began to notice that a significant number of neurological patients, particularly stroke victims and those with Parkinson’s disease, experienced severe depression as secondary symptoms, though not all of them were depressed. Was there a neurological explanation, a pattern of damage to specific areas of the brain—a problem with the wiring—that could explain why some patients were depressed and others were not? Neither the research literature, nor New York’s top neurologists, could provide an answer.
One notable exception was a 1984 study of post-stroke depression by a psychiatrist, Bob Robinson, at Johns Hopkins University. From CT brain scans, Robinson observed that depression was more common in patients with damage to the left frontal lobe. His finding piqued Mayberg’s interest because it suggested that the location of the lesion—the break in the wiring—was somehow implicated in neurological depression. “It was clear that depression involved more than just the lesion. The question was how did the lesion affect the rest of the brain to cause depression? ”
Classical neurologists had to make inferences about how neurological diseases affect brain function by observing a patient’s behaviour and symptoms, and interpreting those clues based on their textbook knowledge and clinical experience. They couldn’t open up a living person’s brain to see how a disease changed the way the whole brain worked. The development of PET-scan technology in the 1970s changed that. It allowed neurologists and brain researchers to observe directly how neurological disorders, like stroke, epilepsy, and Parkinson’s disease, affect brain function and behaviour. (Other imaging technologies, such as X-rays, CT, and MRI scans, show how disease affects the structure of the brain but not how the brain works.)
Mayberg was so excited by the promise of this new scanning technology that in 1985 she took a research position at the Johns Hopkins University School of Medicine PET facility in Baltimore, where she learned how to use PET as a research tool. She used her newfound expertise to help Bob Robinson use PET scans on depressed stroke patients in search of a pattern of abnormal brain activity in stroke patients that would, neurologically speaking, distinguish depressed from non-depressed patients. But stroke patients weren’t ideal subjects because stroke damages different parts of the brain in different people and symptoms vary greatly, making it hard to find enough well-matched patients to do meaningful comparisons.
Mayberg thought that Parkinson’s disease would serve as a better research model because all patients share a common pattern of disease affecting the basal ganglia (structures deep in the brain associated with feelings and movement). Sergio Starkstein, an Argentine neurologist at Hopkins, provided her with a large group of depressed and non-depressed Parkinson’s patients to study. In depressed patients the PET scans showed a striking pattern of reduced brain activity in the paralimbic regions of the frontal lobe that was not seen in the non-depressed patients. (The paralimbic regions are in a lower portion of the cerebral cortex that surrounds the limbic system. The cortex is the outer layer of the brain associated with thinking; the limbic system is the emotional centre located deep inside the brain.) The pattern of reduced paralimbic activity suggested a disruption of the circuit connecting the basal ganglia and lower regions of the frontal lobe, a break in the wiring affecting regulation of mood.
Then, in 1988, Mayberg did a second study comparing the brain activity of depressed and non-depressed patients with Huntington’s disease. It showed exactly the same pattern. In a third study, she scanned a subgroup of stroke patients with damage to the basal ganglia and, again, observed a similar pattern of disconnection.
Finding a common pattern of abnormal brain activity in depressed patients with three different types of neurological disease was a significant breakthrough. In Parkinson’s, Huntington’s, and stroke, however, depression is a secondary condition that results from a primary neurological disorder. How relevant would her PET findings be for the vast majority of depressed people whose depression was unrelated to an underlying neurological disorder?
To find out, Mayberg did PET scans on a small group of patients who were in hospital for severe depression—known clinically as unipolar depression—and who had not responded to drug treatment. Once again the PET scans showed a much lower than normal level of activity in the paralimbic regions connecting the cortical thinking brain and the limbic emotional brain.
In her testing of these four distinct groups of patients, Mayberg had found a consistent biomarker of depression: an uncharacteristically low level of activity in the paralimbic regions. She knew that there was a break in one of the circuits connecting limbic and cortical regions of the brain, making it much harder for the emotional and cognitive regions of the brain to talk to each other. She also suspected that the “short” in this limbic-cortical circuit was only one of many faults in the whole brain network. She had discovered a small island; the next step was to try to expand the map of depression.
In 1991, Mayberg was recruited by the University of Texas Health Science Center at San Antonio, which had just opened a $38-million (U.S.) research imaging facility equipped with more advanced, higher-resolution PET scanners. One visiting scientist dubbed the centre’s diverse brain-mapping activities “a Manhattan project of the mind,” after the crash program to develop the atomic bomb during World War II. The centre encouraged a cross-fertilization of ideas through multidisciplinary collaborations, making it an ideal place for Mayberg to begin a new chapter in her exploration of the human brain.
The Texas medical centre had a big depression clinic, where Mayberg could do PET scans on a broader, more representative population of depressed people than she had studied at Johns Hopkins. Some of them were severely depressed and completely immobilized. “In San Antonio, we saw depressed patients in the real world,” she says. “Their brains were more ‘on.’”
Mayberg ultimately wanted her research to help patients by improving treatment, so she and the psychiatrist Stephen Brannan designed a PET study to tackle two of the most difficult questions in depression treatment: Why don’t antidepressants such as Prozac work right away? Why doesn’t everybody treated with drugs get better?
To find out, they looked at how brain- activity patterns change over time when patients are treated with antidepressants. In this study, fifteen men diagnosed with severe depression were given either a standard dose of Prozac or a placebo. They had PET scans before treatment, and again after one week and six weeks of treatment.
As well as investigating these treatment issues, Mayberg struggled to increase her understanding of how the emotional regions of the brain are altered in depression. From her own PET studies and those of other researchers, she knew that the higher cortical areas of the frontal lobe involved in thinking and attention are typically underactive in depressed people. “We understood that depression turns down cognitive processes in the brain,” Mayberg recalls, “but we couldn’t figure out how the wiring for mood was altered in depression.” To better understand that, she and Mario Liotti, a neurologist and neuropsychologist, set up an ingenious experiment called a “mood challenge study” to look at sadness in healthy people. “A lot of people in the field were adamant that depression and sadness are completely different. We thought, yes, they are different but not so different that they would involve different systems in the brain,” she says.
In the “sad” study, as they called it, eight healthy women were asked to write autobiographical scripts based on memories of terribly sad events. Each woman read her script aloud and a PET scan was then taken to photograph the state of her brain when she was in- tensely sad. The PET camera also took snapshots of each woman’s brain in a neutral state after the sad mood had passed.
Through the PET window, Mayberg saw that as the women became sad, blood flowed down into the limbic emotional centres of the brain and the limbic regions turned a fiery red. “We saw red blobs in the limbic regions,” she says. The emotional brain was revved on high, a hyperactive vortex of misery. As the subjects sank deeper into the pit of sadness, portions of the brain’s right frontal lobe, linked to thought and attention, turned off. During those few minutes of intense gloom, when the sadness was all-consuming, the higher regions of the brain shut down altogether. Then, as the women lifted themselves out of the sad mood, the PET snapshots showed blood had flowed back up to the higher regions, restoring a healthy balance between activity in the cognitive and emotional regions of their brains.
For Mayberg the “sad” study was a revelation that allowed her to see the depressed person’s brain with a new set of eyes. She suddenly saw that the interaction between the emotional and cortical regions was pivotal. “We were watching still images of the battle between emotion and thinking,” she says.
When Mayberg examined the PET scans from the Prozac study, which finished after the “sad” study, she saw that the depressed patients whose mood improved after six weeks of treatment showed a pattern exactly the reverse of the subjects in the “sad” study: brain activity increased in the cortical regions and decreased in the limbic regions. She concluded that mood couldn’t be separated from thinking and attention in the brain. Mood, thought, and attention were tightly wired together, and, in the neural interplay of depression, thought and emotion were like two dance partners badly out of step.
The brain-change patterns of the sad women and the depressed men enabled Mayberg to see the contours of the depressed brain much more clearly. The Prozac study showed how the drug gradually recalibrated the brain’s emotional and cognitive regions to bring them into a healthy balance. After six weeks of treatment, the patients who responded to Prozac felt better and showed increased cortical activity and reduced limbic activity compared to the non-responders.
The Prozac study also produced another fascinating result. While four of the ten men treated with Prozac responded to treatment, four of the five men given placebos got better. PET scans of those who responded showed that metabolism increased in the cortical regions and decreased in the limbic regions after six weeks. The pattern for getting well—reversing the overactive limbic, underactive cortical brain—was seen in both placebo and drug responders.
The placebo results strengthened the evidence about the basic pattern of brain changes required for depression recovery. They were also a reminder that drug treatments aren’t the only therapies that work. In this particular study, a higher percentage of patients responded to placebo than drugs. Did this mean that placebos work as well or better than drugs? No, because the sample was small and other studies show that placebo patients relapse sooner. The PET scans also revealed that patients who recover on antidepressants show additional changes in the brainstem, striatum, and hippocampus. “It could be,” said Mayberg at the time, “that these additional changes facilitated by active drugs are necessary to stay well over the long term. In other words, drug is a placebo-plus. If you respond to a placebo, this may mean that your brain has an inherent capacity to heal itself—but it is likely a short term-effect.”
The tug-of-war between the emotional and thinking regions of the brain that Mayberg perceived in sad and depressed people points to a fundamental fact that in depression there is an imbalance between passion and reason. This relationship between these two faculties is a theme that observers of human behaviour from Plato to Freud have explored. In The Republic, Plato called reason the charioteer who steers the horses (the emotions) and keeps them on track. Without reason to direct them, emotions may run amok, causing the chariot and charioteer to crash. Freud’s psychoanalytic model of human behaviour is similar: the reasonable, conscious ego and irrational, unconscious id are engaged in a permanent conflict, one that lies at the heart of many unresolved psychological problems.
The underlying premise in both approaches is that emotions are inferior to reason and that feelings should be subjugated to reason. Neuroscience shares this traditional view of the brain: the higher cortical areas are associated with thinking, planning, and reasoning, while the lower limbic and subcortical regions are the seat of emotions, drives, and animal instincts. The implicit idea is that emotions originate in the dark basement of the brain and that, as a species, humans should strive to outgrow them.
But that view is gradually changing. In his influential book, Descartes’ Error: Emotion, Reason and the Human Brain, published in 1994, the neurologist Antonio Damasio draws on case studies of patients with specific kinds of brain damage to show how reason and feelings are wired together in the brain and how emotions play a fundamental role in rational behaviour. One such patient, “Eliot,” suffered prefrontal brain damage from a tumour that caused an absence of feeling—an emotional flatness or detachment—but did not affect his intellectual capabilities. It soon become apparent, however, that his practical reason had become so impaired that he’d made a succession of disastrous and uncharacteristic mistakes in business, financial, family, and personal matters. Damasio concluded that reduced emotional capabilities produced irrational decisions and behaviour.
Rather than view reason and emotion as separate faculties, the new neuroscience recognizes how emotion makes an important contribution to reason. In Damasio’s and Mayberg’s integrative models, optimal brain function, or emotional intelligence, depends on the nature of the interconnections and interactions between emotion and reason. In the healthy brain, the key principle is balance, not dominance: emotion and reason co-operate and work together.
In his 1996 book The Emotional Brain, the neuroscientist Joseph LeDoux looks at how the relationship between emotion and thought has changed with the evolution of the brain in different species. He argues that the trend in evolution seems to be towards emotional-cognitive connectivity. There are more connections between cortical and limbic regions in primates than in other mammals, which suggests humans are better able to control their emotions than other species. But there is still an imbalance of power: thoughts can easily trigger emotions, whereas it seems to be more difficult for thoughts to turn emotions off. The trend in evolution may be toward an expanding of these limbic-cortical connections and thus toward a better balance: “If these nerve pathways strike a balance, it is possible that the struggle between thought and emotion may ultimately be resolved not by the dominance of emotional centres by cortical cognitions, but by a more harmonious integration of reason and passion,” Ledoux writes.
The Prozac study in Texas had pointed to a major gap in Mayberg’s research. She hadn’t looked at how or whether psychotherapy changed the way the brain worked. If placebos could change brain activity in depressed people, what effect would psychotherapy have? What had to happen in the brain for psychotherapy to work?
To answer these questions, she would need the help of a psychotherapy expert. “My research is opportunistic,” says Mayberg, who is always looking for suitable collaborators to advance her research. “Who wants to play with me? Who’s up for it? ” In Toronto, where she was recruited as chair of neuropsychiatry at the U of T and Baycrest’s Rotman Research Institute in 1999, Mayberg found the perfect collaborator to put talk therapy to the test in Zindel Segal, head of the Cognitive Behaviour Therapy (CBT) unit at the Centre for Addiction and Mental Health at the Clarke Institute of Psychiatry. CBT is regarded as one of the most effective talk treatments for depression: studies by the National Institute of Mental Health and other groups have found that it’s as effective as medication for treating mild to moderate depression. Segal’s own research has shown that cognitive therapy can reduce the risk of relapse in patients successfully treated for depression by about fifty percent.
In late 1999, Mayberg and Segal launched their first PET study. Patients received one-hour weekly CBT sessions for fifteen weeks. Mayberg went into the study with certain assumptions and expectations. If antidepressants work from the bottom up by suppressing overactivity in the lower limbic regions and then boosting activity in the higher cortical regions, she assumed psychotherapy should work from the top down, increasing activity and waking up the disabled cortical brain, which might then be strong enough to bring the overheated emotional brain under control.
But the PET scans of the CBT patients told a different story. The pretreatment scans showed that the patients had overactive frontal lobes, a pattern that was the reverse of the underactive frontal lobes Mayberg had typically seen in depressed patients. The post-treatment scans showed that the patients who responded to cognitive behaviour therapy had reduced their frontal lobe activity to a normal level after successful treatment. “When we analysed the results, I almost dropped dead. Everything was backwards,” recalls Mayberg. “How could we be that wrong? ”
After the initial shock, Mayberg reexamined the data in more detail and tried to make sense of the results in terms of CBT theory. Cognitive behaviour therapy teaches people to recognize how their view of situations and events is coloured by ingrained negative thoughts, attitudes, and beliefs about themselves, and how that triggers negative reactions, feelings, and moods. “People learn to reappraise their emotions so that they’re not stuck in the same negative thought patterns,” Segal explains. The PET scans showed that a specific area of the frontal cortex, known as area 10, was hyperactive before CBT and then calmed down after successful treatment. “Area ten is activated when people do self-referencing [relating external events, particularly negative ones, to the self] and these people were doing too much of that. They were spinning around in a cycle of negative thoughts about themselves and trying to use the cortex to snap out of it,” says Mayberg. “With CBT, you learn to decrease your self-reference to the things that are negative. It’s a form of rehabilitation of the cortex where patients learn how to turn down the volume.”
The shift from cortical hyperactivity to a normal cognitive state matched the pattern of changes typically seen by CBT clinicians: “Our patients showed very active frontal lobes before treatment. Their minds were racing all the time and they were unable to rein them in,” says Segal. “That hyperactivity was quieted by cognitive therapy, which like any psychotherapy is about helping patients engage in new learning. Cognitive therapy works by actively helping patients change the frontal areas that are essential to effect emotional regulation.”
The results of the CBT study, done with a graduate student, Kimberly Goldapple, caused Mayberg to reappraise and refine her simplified, one-size-fits-all limbic-cortical depression model. “The most fundamental insights come from when it doesn’t go the way you expected,” she says. “The pattern we saw suggested that maybe these people’s brains are different to start with.” Mayberg then began to explore and test the idea that there are a number of distinct patterns of abnormal brain activity among different subgroups of depressed patients. If true, it would have exciting clinical implications. “This raises the possibility that different treatments are appropriate for people with different brain signatures for depression,” says Segal.
Perhaps the state of the brain was telling therapists how it should be treated. If so, Mayberg wanted to decode the message. She re-analysed PET scan data from all the different groups of patients she had studied over the past fifteen years to look for distinct brain signatures of depression. In a major paper published in the British Medical Bulletin in 2003, she identified three basic subgroups—CBT responders, drug responders, and drug non-responders—based on different pre-treatment scan patterns and changes in brain activity in response to treatment.
The CBT responders had a predominantly cortical pattern, in which certain frontal areas were hyperactive and normalized after treatment. Mayberg saw this hyperactive frontal activity, which resulted in agitated, repetitive thoughts, as a maladaptive compensation strategy, an attempt to override a persistent negative mood generated by the lower limbic-subcortical regions. The drug responders had a combined limbic-cortical pattern in which overactive limbic areas were suppressing cortical areas; successful treatment brought the limbic and cortical regions into balance. The drug non-responders had a more pure limbic pattern, in which the limbic areas were dominant and disconnected from the severely disabled, inactive cortical regions.
Each of the three subgroups Mayberg identified has its own distinctive pattern of abnormal brain activity that could potentially serve as a biomarker, or indicator, to improve diagnostic accuracy and guide treatment selection for individual patients. “One needs to know the state of the brain in depression and these are three distinct brain compensation patterns,” she says. “Short of a cure for depression, the next best thing is to say I want to make an educated and evidence-based decision about how to optimally treat the patient. Are there any objective data that can help me to do that? A model like this gives you the best chance.”
The model of depression that Mayberg reconstructed and refined in Toronto illustrates the potential of brain imaging as a tool to help optimize the diagnosis and treatment of depression in the future. This is a clinically oriented research model that she will continue to test and modify, but already Mayberg’s new map of depression has helped to move the field onto a more evidence-based footing.
In the spring of 2003, Mayberg undertook a bold experiment to test the clinical viability of her model on a group of severely depressed patients who had failed repeatedly to respond to a variety of antidepressants and electroconvulsive therapy. “These are people who are so sick that none of the available therapies work. They live in a depressive episode that never ends,” she says. In collaboration with Dr. Andres Lozano, a world-renowned neurosurgeon at Toronto Western Hospital, she pioneered the use of a neurosurgical technique called Deep Brain Stimulation (DBS) to treat five severely depressed patients. Lozano had successfully used DBS to treat patients with Parkinson’s disease and other disorders.
Deep Brain Stimulation is a neurosurgical procedure that uses a device similar to a cardiac pacemaker to send constant electrical stimulation to targets deep inside the brain. Brain imaging is used to guide the surgeon in placing electrodes in a precise target area and to monitor the effects of electrical stimulation on the patient’s brain activity following the procedure. Unlike traditional psychosurgery, DBS is reversible and adjustable, since the strength and location of the stimulation can be modified after the procedure.
In these five patients, electrodes were implanted in white-matter fibres that connect to the cortex and a limbic region known as area 25. The strategy was to interrupt a faulty connection between the overactive limbic area 25 and an underactive cortical region (known as area 9), a dysfunctional loop that Mayberg first observed in her Prozac and “sad” studies in Texas. “If you can’t talk the brain into equilibrium, or drug or shock it into balance, maybe you can jump-start it with an appropriately placed pacemaker,” she says. “We believe that stimulating inhibitory cells in this area jams the circuit and has the effect of turning down limbic overdrive in [area] twenty-five. Turning down twenty-five releases limbic control over the cortical regions and allows the cortex to come back on-line.”
Mayberg and Lozano will be following these patients for one year to assess the effectiveness of DBS as an alternative treatment. “We’ll see whether their behaviour changes in the way our hypothesis predicts. The results are very encouraging so far. Some patients are doing remarkably better,” she says. “The question is, will the change be sustained long-term? ”
Don’t expect to be able to go to your doctor or therapist for a PET scan today. “Helen Mayberg’s work is amazing. But there are a lot of things that need to be done before we can use this as a clinical tool,” says Dr. Trevor Young, physician-in-chief at the Centre for Addiction and Mental Health. “Her findings would need to be replicated with a larger sample. PET is expensive and extremely cumbersome. It won’t be widely available to the public. If there are alternatives to PET, other brain imaging techniques or neuropsychological tests, that could make it more broadly available.”
The map isn’t done yet, but neither is Mayberg. Her model provides a template for researchers and clinicians to build on. One can envisage many different ways in which brain imaging and biomarkers could be used to optimize diagnosis and treatment of depression in the future. “Imaging results could help you decide whether to choose medication or psychotherapy as a starting point,” says Dr. Sidney Kennedy, psychiatrist-in-chief, University Health Network in Toronto. Once drug or talk therapy begins, brain scans could be used to help monitor and manage treatment by tracking changes in brain activity over time. Right now patients often have to try many different medications before finding one that works. Or, after many months or years of trial and error, they may find that none is effective. Brain scans during treatment could show at an earlier stage whether or not a particular medication is working and help the clinician to adjust the dosage or recommend alternatives. Similarly, in talk therapy, scans could be used to assess whether or not a particular approach is working and guide the psychotherapist in tailoring the type of therapy to fit the needs of a particular patient. For those severely depressed patients who don’t respond to talk or drug therapy, PET scans could help to refine and assess the viability and safety of experimental treatments, such as Deep Brain Stimulation, that alter brain circuitry directly.
Brain imaging could also be used to prevent depression. People who have experienced at least three prior depressive episodes have about a seventy-five percent chance of relapse, while those with no previous depression history have about a twenty-five percent relapse rate. Mayberg’s mood challenge experiments suggest that certain depression trait markers in the brain could be biomarkers of relapse risk, early warning signs of vulnerability for further episodes of depression. Once a person is successfully treated for depression, periodic brain scans could be done to assess relapse risk and guide maintenance therapy to prevent recurrence.
PET scans could also help people at risk due to a family history of depression, early-life trauma, or depressive personality traits. Just as there are tests that can help people at risk for heart disease take steps to prevent a heart attack, brain imaging could identify biomarkers of illness vulnerability in people at risk for depression and help design preventive treatment. “It’s bad for your brain to get sick,” says Mayberg, noting that early intervention helps to minimize the cumulative impact of repeated episodes of depression on the brain. “The way one handles vulnerability to stress can be retrained through therapy,” says Mayberg. “If your brain learns how to handle stress in a better way, this may prevent depression if you are at risk because of early trauma or genes, or protect against relapse if you’ve recovered from depression.”
Depression is a condition that involves disturbances of both mind and mood, in which the self becomes a house divided by an internecine war between passion and reason. Helen Mayberg’s neuroanatomical model of depression, which graphically displays imbalances between emotional and cognitive brain regions, makes our understanding of depression less fuzzy. Her map of depression circuits in the brain charts a new path to guide the healing of divided selves towards a stronger, healthier balance in which thoughts and feelings interconnect in a complementary union. Just as humans as a species can choose between the downward spiral of mutual destruction and a higher evolutionary path towards peaceful co-existence, the depressed brain can adapt to a new, healthier state by forging stronger links between the emotional and cognitive regions. “Our emotions are hard-wired to affect our thinking and vice-versa,” says Mayberg. “When there’s an imbalance, the goal of therapy is to restore a stable equilibrium: a healthy choreography of emotion and thinking.”