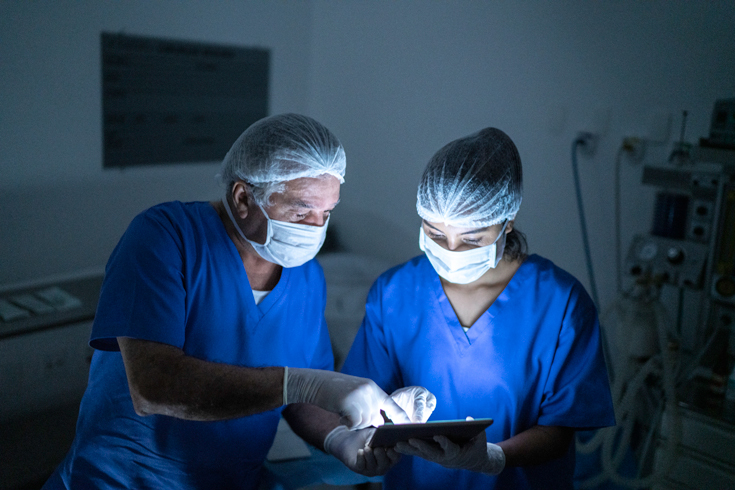
On March 16, doctors at Perth and Smiths Falls District Hospital, in eastern Ontario, were preparing for an influx of COVID-19 patients. It was the fifth day of the pandemic, and they didn’t know what lay ahead. News from other countries was nightmare inducing: Italian doctors were triaging desperate patients in an attempt to direct available ventilators to those with the highest chances of survival. Like physicians around the world, doctors in Perth and Smiths Falls worried there wouldn’t be enough ventilators when the influx came.
That’s when Alain Gauthier, a general-practice anesthetist at the hospital, pulled together several old anesthesia machines, used to deliver oxygen to patients in surgery, from around the facility and reconfigured them into ventilators. Then, using tips he found in a YouTube video posted by a Detroit emergency room physician two days earlier, Gauthier, who has a PhD in respiratory mechanics, rejigged each of the machines so that one device could ventilate two patients at the same time.
The idea came as hospitals around the world were preparing for the arrival of COVID-19 in their wards and fearing that this new respiratory disease would bring surges of critically ill patients who couldn’t breathe. Knowledge of SARS-CoV-2, the new coronavirus causing the pandemic, was not yet three months old; doctors were learning about the virus and writing protocols for its treatment in real time, with little evidence to guide them other than scattered reports from the hardest-hit hospitals around the world.
The shared-ventilation method had been studied in theory but used successfully on humans only once, during a mass-casualty event—the 2017 Las Vegas shooting. (Later in March, it was reported that some doctors in New York City and Italy were also using the technique to treat COVID-19 patients.) Having two people on one ventilator is not ideal: the machine’s settings are designed to be calibrated to one set of lungs. Even in the rare case where two patients’ needs are the same when they start on a ventilator, there’s no guarantee that their conditions will progress synchronously—one may deteriorate while the other recovers, and the distribution of oxygen to each patient would become unsuited to their needs. In March, several American organizations cautioned against placing multiple patients on a single mechanical ventilator, arguing that it was better to use the machine on the patient who was most likely to benefit.
But split ventilation could offer the possibility of life for a patient who would otherwise die, and this is what frightened physicians and patients around the world needed. Perth doctors began fielding calls from health care workers around the world who were interested in macgyvering their own anesthesia machines to increase ventilator capacity. By late March, as New York state surpassed China’s Hubei province in the number of people infected, the US Food and Drug Administration gave emergency authorization that ventilators could be modified to serve multiple patients, what’s known as an “off-label” use. In Canada, the number of people requiring ventilation has remained below the number of available machines, and no organization has issued statements about the protocol on sharing ventilators in case of a shortage.
Eight months into the pandemic, much has changed about what we know of this disease. Mechanical ventilators are no longer used as quickly in a patient’s course of illness as they were a few months ago. But uncertainty over the future remains. Case counts are galloping upward across Canada, just like in the United States and Europe. Around the world, we’re still trying to figure out how best to treat this perplexing disease, which can take a toll on every body system with little predictability. The question raised by the ventilator dilemma is one that doctors still have to grapple with for the foreseeable future: What is the standard of care when there’s a limited body of evidence but a growing number of patients in need of health care resources?
For the past three decades, evidence-based medicine has been central to the provision of health care. It’s the philosophy that physicians should make decisions about a person’s care according to a body of verified empirical evidence about their illness. At the top of the evidence hierarchy are randomized controlled trials. For these trials, investigators test a therapy against a control—a new drug or device or technique against a well-established existing one or a placebo. Patients with similar characteristics are randomly assigned to either arm of the trial. Investigators analyze the results, pass them on for peer review, and finally publish the findings in scientific journals. Just below these on the hierarchy are well-designed trials without randomization, followed by case series that track a group of people with similar diagnoses or treatments, then by anecdotal reports from clinicians, and then, at the very bottom, by expert opinion based on theory.
Very little of that evidence pyramid existed for the treatment of COVID-19 when the disease landed in Canada. Doctors couldn’t rely on their clinical judgment in the way they had been trained to do. They couldn’t just rush in to care for a patient who couldn’t breathe: they needed to stop and don personal protective equipment first. By late March, Fareen Zaver, an emergency room physician in Calgary, said COVID-19 was “changing 100 percent of our regular, normal practice.” When the city had its first cases, it wasn’t clear what would help patients and what would make them worse. That was true for everything from treatments to policies about when patients should be separated from their families in order to prevent further spread. “I think that’s the part that all of us are so fearful of: What kind of harm is this going to potentially cause our patients?” Zaver said.
These rapid changes, which continue to play out as the pandemic morphs and deepens in different jurisdictions, have raised ethical concerns about when and how to use new therapies on patients—concerns that have been further complicated by politicians, the press, and social media.
Building a base of evidence takes time. Generally, when a novel treatment comes to market for a particular illness, it goes through a series of trials to test its safety and efficacy, then it is gradually released to market after passing through various checks and balances.
COVID-19 left no time for all of that. Faced with desperate patients and no proven treatments, clinicians on the ground began sharing information over social media about what they were seeing in hopes that it could save the lives of patients elsewhere in the world. Physicians and other health care workers tweeted out tips learned over the course of a shift—everything from the physical set-up of the ICU to CT images of the disease’s brutal takeover of a patient’s lungs. Medical publications, such as the Journal of the American Medical Association (JAMA), The Lancet, and The New England Journal of Medicine (NEJM), dropped their paywalls and began quickly publishing reports by physicians based in places like Wuhan, Lombardy, and New York City. Throughout 2020, there’s been an ongoing flurry of preprint papers, which are released publicly without undergoing the standard peer-review process whereby authors’ work is scrutinized by a panel of experts. Expediency trumped scrupulousness because, around the world, patients needed help yesterday.
Doctors try novel treatments all the time, especially when knowledge about a condition or patient is incomplete, says Todd Campbell Lee, an infectious-disease physician at McGill University. But the pandemic amplified the sense of urgency. The result was what Lee calls “a kind of random care” in lieu of a set standard for care. “It may lead to better outcomes for some, and it may lead to worse outcomes for some.”
Did random care save lives? Did it cause harm? It took time to figure out which treatments helped and which didn’t, and there’s still much we don’t know. In this time, physicians and scientists worked their way up from the bottom of the evidence hierarchy. Existing therapies and devices were repurposed for use against the virus. A treatment—a drug, a device, something as simple as placing patients on their stomach in an ICU—was tried at one institution and reported via social media or through preprint publications. When anecdotes of success emerged, these nuggets were boosted in the press and sometimes by politicians—often without acknowledging the many limitations of preliminary research. Other physicians and patients took notice because they wanted everything, anything, something that might help.
This is the story of hydroxychloroquine. The drug, commonly used to prevent malaria, got a big boost from a case report out of France in late March: twenty patients received hydroxychloroquine daily and, after six days, had a greater reduction in viral load than did untreated patients from another centre and those who refused the drug. Desperate physicians began ordering it for patients. US president Donald Trump praised it as a “game changer” and authorized his government to purchase and stockpile 29 million doses. Clinicians started to enrol patients in randomized trials, and Health Canada approved the enrolment of Canadian patients.
Then came the first twist: a massive May 22 study in The Lancet suggested that hydroxychloroquine was dangerous for COVID-19 patients—it contributed to heart problems and appeared to increase the risk of in-hospital death. The World Health Organization (WHO) hastily suspended its trial of the drug. But the criticism, too, was found to have been hasty. As it turned out, the reviewers had not been able to conduct an independent audit: Surgisphere, the American company that had provided the data analysis for the study, had never made its data set available to them. The paper’s damning conclusions had come from numbers that could not be verified, and The Lancet retracted it.
Hope that the drug may save patients with COVID-19 has faded. In July, Lee and colleagues from the University of Minnesota published, in Annals of Internal Medicine, the results of their randomized controlled trial looking at hydroxychloroquine in people with COVID-19 symptoms outside of hospital. The therapy did not lead to faster symptom improvement, the study showed. To date, at least three of the trials investigating hydroxychloroquine in patients with COVID-19 have been halted because the evidence collected so far shows no benefit. This includes the hydroxychloroquine arm of the WHO’s Solidarity Therapeutics Trial, which Canadian institutions had been contributing to. (The trial continues to investigate two therapies: remdesivir and lopinavir/ritonavir with interferon beta-1a.) On November 9, after another randomized trial failed to support the use of hydroxychloroquine to treat COVID-19 in hospitalized patients, Michael S. Saag wrote, in an editorial in JAMA, that the “clear, unambiguous, and compelling lesson from the hydroxychloroquine story for the medical community and the public is that science and politics do not mix.”
The hydroxychloroquine buzz almost killed the randomized clinical trials designed to show what the benefits or risks of this treatment would be. The frenzy around the drug was taking hold before scientists even had a chance to make up their minds about it, and it became easier to give patients the drug off-label, without scientific or ethical oversight, than to take the time to enrol patients in a trial. Patients themselves asked to skip the trial process and receive the drug. The experience “was a complete failure of the scientific method,” Zain Chagla, an infectious-disease physician at McMaster University, said at the time.
Therapies should be subject to rigorous study before being rolled out across the population outside of clinical trials, Chagla said. “Giving the drug completely off-label, without consent of the patient, without actually knowing it works, ethically, that’s horrible. It just reversed the way we generate evidence that refers to the scientific method that we’re doing.”
While some randomized trials for treatments have caught up in recent months, the early days of the pandemic saw COVID-19 patients around the world being administered drugs that may have been more dangerous, more expensive, or have had more side effects—but they were given off-label and not tracked in randomized trials. Instead, the medical community should have prioritized randomized controlled studies, which will accrue evidence in the strongest way, said Chagla.
Ideally, new therapies are offered within the context of an approved clinical trial, but that’s not always possible, and it’s especially difficult during the pandemic, said Sally Bean, an ethicist at Toronto’s Sunnybrook Hospital. Sometimes, she added, off-label use is justified: “The stakes are so high when we’re dealing with the potential for patients to have serious morbidity or mortality from this disease. It’s a high-pressure situation from our standpoint.” According to Bean, it falls to a physician’s clinical judgment, in discussion with a patient and their family, to ascertain if the potential benefits exceed the risks in a given case.
In May, Health Canada introduced an interim order for clinical trials related to medical devices and drugs for the treatment of COVID-19. The change is designed to speed up the trial process by cutting some of the administrative burden around trials and making it easier to enrol people in a clinical trial. There are currently about seventy Health Canada–authorized trials underway in Canada.
“It’s a recognition that things are going to be a little bit different in terms of clinical research in the time of COVID-19,” said Chagla.
COVID-19 is frightening, fast moving, and fickle, and it has turned the world inside out as we wait anxiously for a treatment we needed months ago. But, to find a treatment that works, health care workers and patients need to test treatments that may not be as successful. That, unfortunately, is a given, and to do it ethically, physicians and researchers need to explore treatments in the context of well-designed clinical trials. This is the only way we will know what works, what doesn’t, and in whom.
A few weeks after the hydroxychloroquine paper was pulled from The Lancet, the medical world was abuzz about another old drug repurposed for coronavirus. A group from the University of Oxford reported that dexamethasone, an inexpensive steroid used to treat different inflammatory conditions, had reduced deaths by one-third in ventilated patients, and by one-fifth in patients receiving oxygen, in a large ongoing trial. The UK’s chief scientific adviser, Patrick Vallance, said in a statement: “This is a ground-breaking development in our fight against the disease, and the speed at which researchers have progressed finding an effective treatment is truly remarkable. It shows the importance of doing high quality clinical trials and basing decisions on the results of those trials.”
The announcement came via press release, with no specifics on the study details. Physicians had a choice: give dexamethasone or not, based on a study not yet published.
The full results of the study, known as Recovery, came out one month later, in the NEJM, showing that dexamethasone reduces mortality among the very sickest COVID-19 patients, those who were already receiving mechanical ventilation or oxygen. In an accompanying editorial, Anthony Fauci and H. Clifford Lane, of the US’s National Institute of Allergy and Infectious Diseases, called scientifically robust clinical research “the quickest and most efficient pathway to effective treatment and prevention strategies for patients with COVID-19.” Chagla said Recovery demonstrates what researchers have been calling for since the beginning of the pandemic: in the absence of evidence, we should practice medicine in a way that allows researchers to collect it. “We’ve all learned our lesson that placebo-controlled trials are the gold standard of care. And I think we really, really need to prioritize them as clinical studies on go.”
Dexamethasone is now the standard of care for critically ill hospitalized patients with COVID-19 because, if administered within the right window, it reduces their rates of mortality. It’s worth noting that, in the six days after the initial press release about the Recovery results but before the study was published, some hospitals in the United States increased demand for dexamethasone by 610 percent.
It’s also worth noting that, this fall, one of the world’s loudest early cheerleaders for hydroxychloroquine became one of the world’s most famous COVID-19 patients. Trump was hospitalized at Walter Reed National Military Medical Center in October. He did not receive hydroxychloroquine. Instead, he was treated with dexamethasone and remdesivir—treatments with more data behind them. The latter speeds recovery time in hospitalized COVID-19 patients, according to a large randomized controlled trial that opened in February and took place across sixty different sites worldwide.
On October 2, Trump also received an experimental monoclonal antibody cocktail, REGN-COV2, made by biotech company Regeneron. Three days prior, the company had released a press statement that reported the first data from a trial involving 275 people randomized to placebo, low-dose monoclonal antibody treatment, or high-dose treatment. The greatest treatment benefit was in patients who had not yet mounted their own effective immune responses. Additional details have not been published.